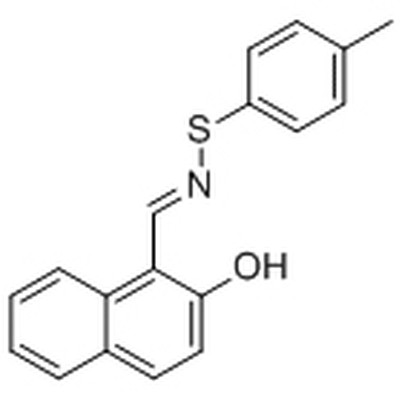
What is the solubility of S - 64315 in water?
Leave a message
Hey there! I'm a supplier of S - 64315, and today I wanna chat about one of the most common questions I get: What is the solubility of S - 64315 in water?
First off, solubility is a big deal when it comes to chemicals like S - 64315. It determines how well the compound can dissolve in a particular solvent, in this case, water. This property is super important because it can affect how the chemical is used, stored, and even how it reacts with other substances.
S - 64315 is a compound that has shown a lot of potential in various research areas. But its solubility in water isn't as straightforward as you might think. Unlike some chemicals that dissolve easily and completely in water, S - 64315 has a relatively low solubility.
The solubility of a chemical in water depends on a bunch of factors. One of the main ones is the chemical structure of the compound. S - 64315 has a complex molecular structure, with certain groups that don't interact well with water molecules. Water is a polar molecule, which means it has a positive and a negative end. For a compound to dissolve well in water, it usually needs to be polar or have polar groups that can form hydrogen bonds with water molecules. But S - 64315 has non - polar regions in its structure, which makes it less likely to dissolve in water.
Another factor is temperature. Generally, an increase in temperature can increase the solubility of many chemicals in water. This is because higher temperatures give the molecules more energy, allowing them to break apart and mix more easily. However, for S - 64315, the effect of temperature on its solubility in water is limited. Even when you raise the temperature, the solubility doesn't increase significantly.
So, what does this low solubility mean for those who are using S - 64315? Well, if you're doing research that requires S - 64315 to be in an aqueous solution, you might run into some challenges. You can't just dump it into water and expect it to dissolve completely. You'll probably need to use some tricks to get it into solution.
One common approach is to use co - solvents. A co - solvent is a second solvent that can be mixed with water to increase the solubility of the compound. For example, you could use a small amount of an organic solvent like ethanol or dimethyl sulfoxide (DMSO) along with water. These organic solvents can interact with the non - polar parts of S - 64315, helping it to dissolve better in the overall solution.
Now, let's compare S - 64315 with some other related compounds. Take MIK665 CAS No.: 1799631 - 75 - 6. MIK665 has a different chemical structure, and its solubility in water might be different from that of S - 64315. Some compounds in the same family as S - 64315 might have better or worse solubility depending on their specific molecular features. For instance, FSEN1 CAS No.: 862808 - 01 - 3 could have a solubility profile that's either more or less favorable in water compared to S - 64315. And S63845 CAS No.:1799633 - 27 - 4 also has its own unique solubility characteristics.
In research settings, understanding the solubility of S - 64315 is crucial for accurate experiments. If the compound isn't properly dissolved, it can lead to inconsistent results. For example, if you're testing the biological activity of S - 64315 in a cell culture that requires an aqueous environment, the undissolved particles might not reach the cells effectively, giving you false - negative or inaccurate data.
As a supplier of S - 64315, I've seen a lot of researchers struggle with solubility issues. That's why I'm always here to offer advice and support. I can provide some tips on how to dissolve S - 64315 as effectively as possible. I've also worked with different research groups to develop optimized protocols for handling this compound in aqueous solutions.
If you're in the market for high - quality S - 64315, I'm your go - to supplier. I can provide you with detailed information about the compound, including its solubility data and any other technical details you might need. Whether you're a small research lab or a large pharmaceutical company, I can meet your needs.
If you're interested in purchasing S - 64315 or have any questions about its solubility or other properties, don't hesitate to reach out. We can have a chat about your specific requirements and how I can help you get the most out of this compound.
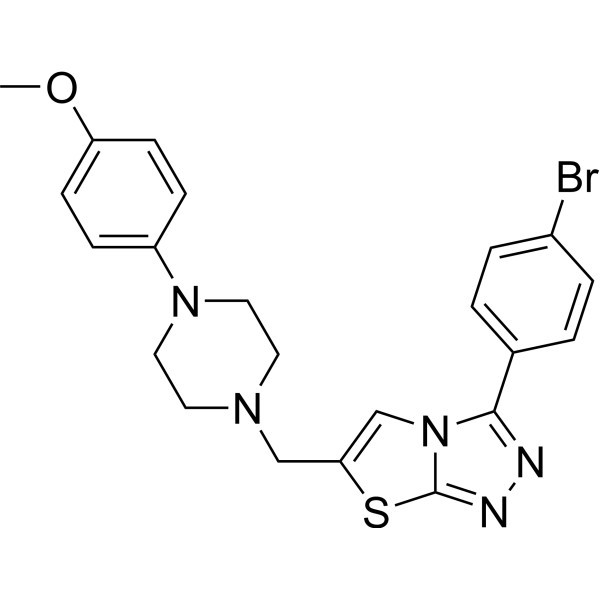
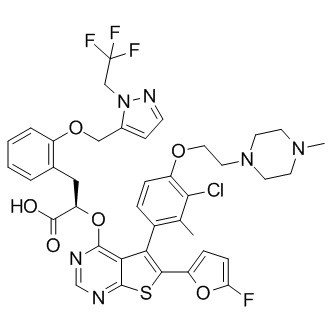
In conclusion, the solubility of S - 64315 in water is relatively low due to its chemical structure and other factors. But with the right techniques and support, you can still use it effectively in your research. So, if you're looking for a reliable source of S - 64315 and need some guidance on solubility and handling, I'm here for you.
References
- General chemistry textbooks on solubility and chemical properties.
- Research papers on the properties and applications of S - 64315.